BY Safura Abdool Karim —
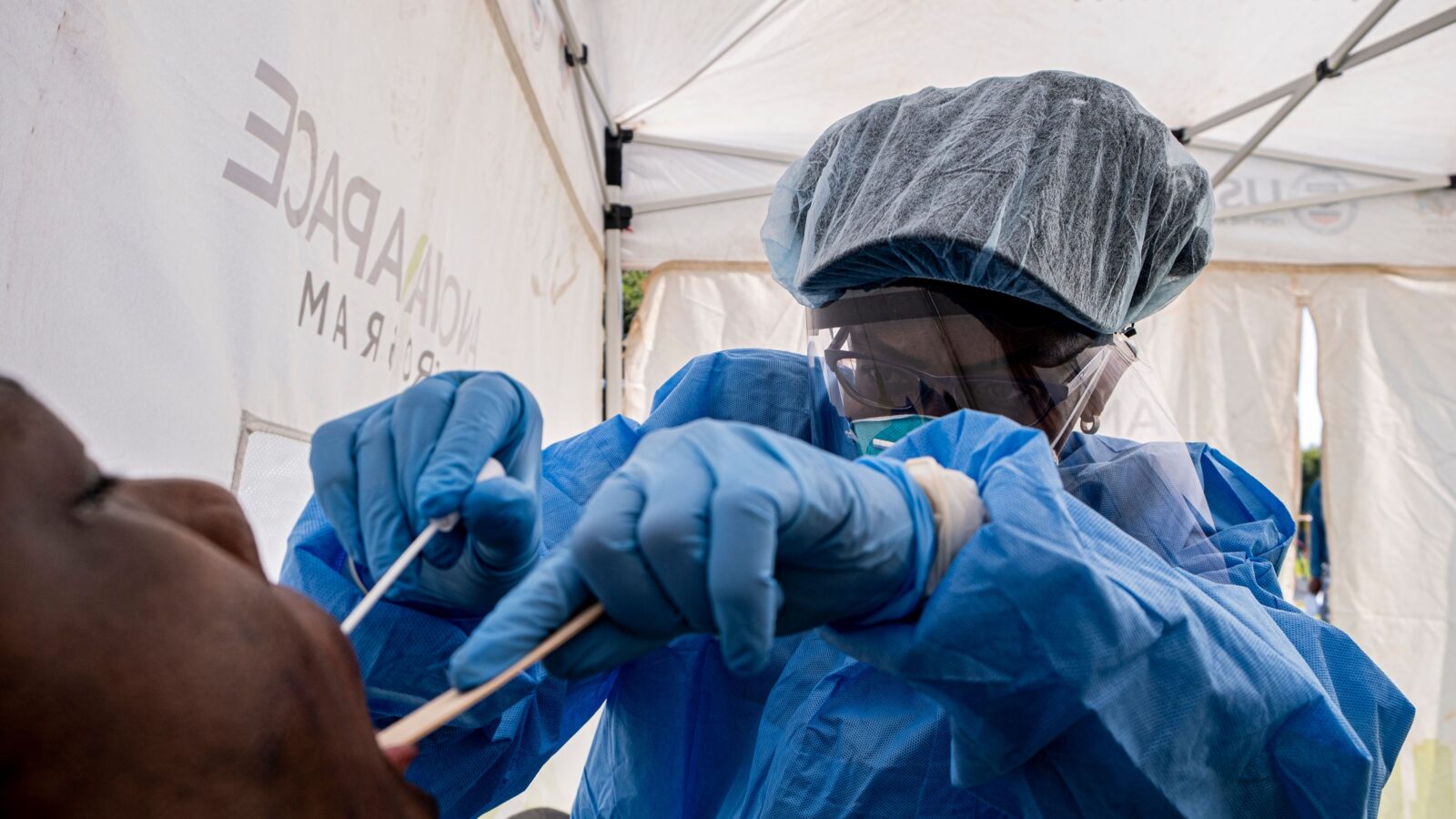
Within the span of 10 days, regulators in the United Kingdom and the United States granted emergency use authorization for the Covid-19 vaccine made by Pfizer and BioNTech.
This vaccine, reported to be 95% effective, is now being rolled out across both countries. And as I write this, the U.S. is poised to approve a second Covid-19 vaccine, this one made by Moderna.
It will likely take far longer for this vaccine to be available in sub-Saharan Africa, partly because its use was approved with emergency use authorizations instead of the developers pursing full licenses.
Previous efforts to expedite Covid-19 vaccine approval, such as Operation Warp Speed, were met with concerns about safety. In response, the largest vaccine developers pledged to submit their vaccine candidates for approval only after passing Phase 3 trials showed a vaccine’s effectiveness.